|
|
[Last Modified: ] |
|
|
|
| [Taenia solium] [Taenia spp.] |
Cysticercosis:
Antibody Detection
CDC's immunoblot assay with purified Taenia solium antigens has been acknowledged
by the World Health Organization and the Pan American Health Organization as the
immunodiagnostic test of choice for confirming a clinical and radiologic presumptive
diagnosis of neurocysticercosis. CDC's immunoblot is based on detection of
antibody to one or more of 7 lentil-lectin purified structural glycoprotein antigens from
the larval cysts of T. solium in an immunoblot format. It is 100% specific
and has a sensitivity superior to that of any other test yet evaluated. Serum
specimens from 97% of parasitologically confirmed cases of cysticercosis have detectable
antibodies. No serum samples from patients with other microbial infections react
with any of the T. solium-specific antigens. The most important factors
identified as determining positive immunoblot reactions are the numbers and stage of
development of cysticerci. Cumulative clinical experience has confirmed that in patients
with multiple (more than two) lesions, the test has more than 95% sensitivity.
Seropositivity in biopsy-confirmed patients with single, enhancing parenchymal cysts was
<50%; in clinically defined patients with a single cyst but who were not biopsied,
sensitivity was 70%. Seropositivity in serum and CSF of patients with multiple but only
calcified cysts was 82% and 77%, respectively. In all patients, regardless of their
clinical presentation, the immunoblot assay is slightly more sensitive in serum than in
CSF specimens: consequently, there is no need to obtain CSF solely for use in the
immunoblot assay.
CDC's immunoblot is both more specific and more sensitive than enzyme immunoassay (EIA) systems with which it has been compared. Lack of specificity has been a major problem in most EIAs because of cross-reacting components in crude antigens derived from cysticerci; these components react with antibodies specific for other helminthic infections, especially echinococcosis and filariasis. Most partially purified fractions evaluated in an EIA appear to have lower sensitivity than crude antigens and do not necessarily achieve higher specificity. Assays employing crude antigens for the detection of antibody are not reliable for the identification of this disease; all positives and any negative strongly suspected of cysticercosis should be confirmed by immunoblot. Currently available antibody detection tests for cysticercosis do not distinguish between active and inactive infections and thus have not been useful in evaluating the outcomes and prognoses of medically treated patients. Both CDC's immunoblot and an EIA are commercially available in the United States.
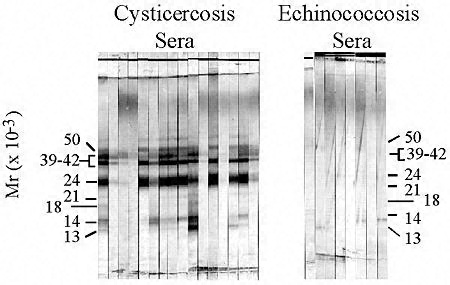 |
| A |
Typical antibody reactions in CDC's immunoblot for cysticercosis. Individual sera from patients with either cysticercosis or echinococcosis were analyzed using the immunoblot for cysticercosis.
Cysticercosis-specific antibodies react with glycoproteins derived from T. solium cysts. The positions of the seven diagnostic glycoproteins are marked and designated according to their relative mobilities in SDS-PAGE. Sera from patients with cysticercosis react with at least one of the cysticercosis-specific proteins, whereas sera from patients with echinococcosis do not react with any of the seven diagnostic proteins.
Reference:
Tsang VCW, Wilson M. Taenia solium cysticercosis: an under-recognized but serious public health problem. Parasitol Today 1995;11:124-126.
|
|||